Translational quality control
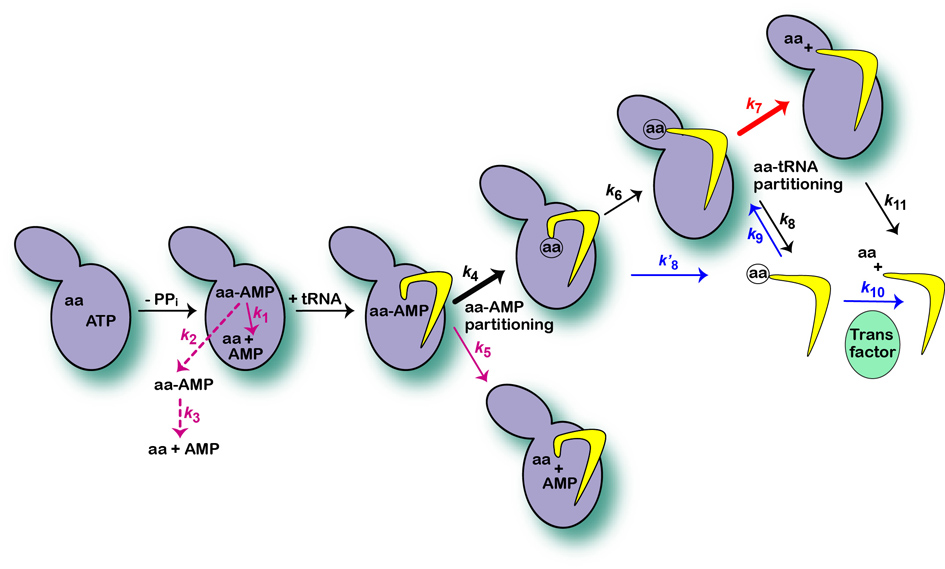
Aminoacyl-tRNA synthetases (aaRSs) catalyze ATP-dependent covalent coupling of the cognate amino acid-tRNA pairs. aaRSs that are unable to discriminate well against non-cognate amino acids in the synthetic reaction alone, have developed the additional editing activities. The synthetic site promotes activation of the amino acid and its subsequent transfer to the tRNA. Within this site hydrolysis of the non-cognate aminoacyl-AMP intermediate may take place (pre-transfer editing). Post-transfer editing is placed in a separate protein domain where misaminoacylated tRNAs are rapidly hydrolyzed.
IleRS, ValRS and LeuRS are used as model enzymes to investigate the mechanisms by which specificity against amino acids is exercised in the synthetic and editing sites of the bacterial and eukaryotic enzymes. Using pre-steady and steady state kinetics we investigate the contribution of kinetic partitioning between synthetic and editing pathways to the aminoacylation accuracy. Evolution of editing and its role in cell physiology under various stress conditions is explored by various genetic methods. We showed that non-canonical amino acids, natural by-products of metabolism, may present the main physiological target of translational quality control (like LeuRS and norvaline). Thus, a potential of various natural non-proteinogenic amino acids to compromise the fidelity of protein synthesis is explored and link to protein misfolding diseases/responses are sought. Finally, we characterize the capacity of translational quality control to act against artificial amino acids to provide better understanding of the principles that govern incorporation of the synthetic amino acids into designer proteins.
Selected Publications:
The physiological target for LeuRS translational quality control is norvaline.
EMBO journal. 33 (2014), 15; 1639-1653
Cvetesic Nevena; Palencia Andres; Halasz Ivan; Cusack Stephen; Gruic-Sovulj Ita.
Synthetic and Editing Mechanisms of Aminoacyl-tRNA Synthetases.
Topics in current chemistry. 344 (2014) ; 1-42
Perona John J; Gruic-Sovulj Ita.
Kinetic Partitioning between Synthetic and Editing Pathways in Class I Aminoacyl-tRNA Synthetases Occurs at Both Pre-transfer and Post-transfer Hydrolytic Steps.
The Journal of biological chemistry. 287 (2012), 30; 25381-25394
Cvetesic Nevena; Perona John J.; Gruic-Sovulj Ita.
Partitioning of tRNA-dependent Editing between Pre- and Post-transfer Pathways in Class I Aminoacyl-tRNA Synthetases.
The Journal of biological chemistry. 285 (2010), 31; 23799-23809
Dulic Morana; Cvetesic Nevena; Perona John J; Gruic-Sovulj Ita.